Written by Nina Lauterbach, PhD (Field Application Scientist, Omixon)
Animals, and us humans, are daily under attack by viruses and other pathogens. Fortunately, we are equipped with the Major Histocompatibility Complex (MHC) as an efficient and adaptable fighting mechanism to deal with these threatening events. This complex is encoded by the most polymorphic genes of the vertebrate genome, known as the Human Leucocyte Antigen (HLA) system in humans.The MHC gene variability in humans is thought to be selectively driven by pathogens, as pathogen-mediated selection promotes allelic diversity in the host as defense mechanism (1). It has been proven that the polymorphic character of the MHC is correlated to the resistance against parasites and viruses, as some alleles have a protective effect (2-8). In line with this, MHC heterozygous individuals have an enhanced resistance to infectious diseases compared to MHC homozygous individuals, since two different alleles can present a wider variety of peptides to the immune system (9-14). In short, it all comes down to one simple conclusion; the more HLA diverse we are, the more efficient our immune system is!
Interestingly, it seems that we are subconsciously guided by this immunologic advantage of MHC variation since we try to find a partner who is genetically different from ourselves. Us humans as well as animals use odor and visual cues to select an MHC dissimilar partner (15-19), and we even choose a perfume to enhance our own MHC genetic profile (20-22). In this subconscious selection process, heterozygous partners are preferred, and not only because they are considered more healthy and thus potentially better providers, but having a heterozygous partner also increases the odds of heterozygous offspring (23, 24). In line with this, it has been shown that sexual attractiveness of women towards their partner decreases when their partner is MHC similar, and these women also reveal to be sexually less satisfied, and are more likely to show infidelity, when compared to women that have an MHC dissimilar partner (19, 25). Also, the wish for children is lower in women with an MHC similar partner.
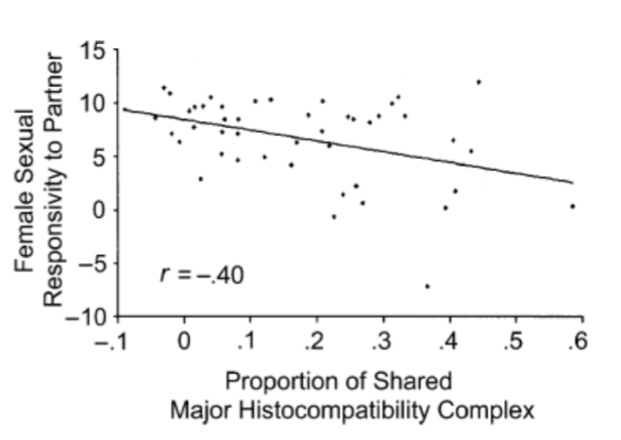
Association between sharing of major histocompatibility complex (MHC) alleles and females’ sexual responsivity to their partners (Adapted from Garver-Apgar et al. 2006 (25)).
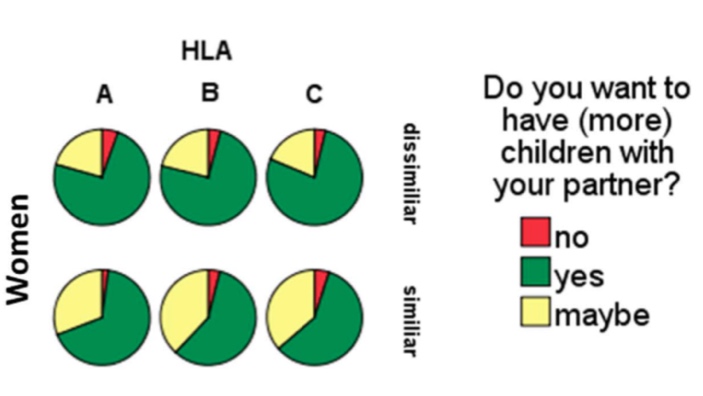
The impact of HLA similarity on the wish for children of women (Adapted from Kromer et al. 2016 (19)).
Males however, seem to be less choosy, since men’s sexual responsiveness is not different when being with an MHC similar partner as compared to being with an MHC dissimilar partner, underlining that mostly women are instinctively pressured in selecting an MHC diverse mate. Interestingly, it has been proposed that the MHC related mate preferences of women can be changed by usage of the contraceptive pill. In 1995, researchers in Switzerland performed the so called “sweaty t-shirt” experiment where female students were asked to rate the scent of t-shirts worn by male student for attractiveness. This demonstrated that females prefer the odor of males with different MHC genes from their own. However, when looking at the female students that were using the pill it seemed that the pill reversed this situation. Females using the pill actually preferred the scent of men with similar MHC (26).
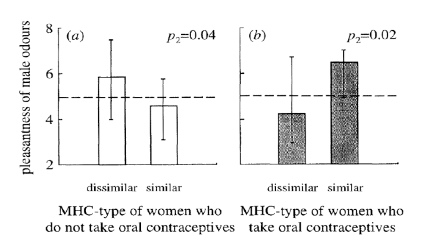
Average score per male by females who are similar or dissimilar on their MHC. The odors were judged by females who did not take oral contraceptives and judged by females who take the contraceptive pill (Adapted from Wedekind et al. 1995 (26)).
Having said this, it seems that the natural choice for an HLA diverse partner lies not only in the higher possibility of having a healthy spouse and offspring, but also lies in the regulation of the reproductive system itself. An increased number of epidemiological studies indicate that incompatibility between partners, rather than compatibility, seems necessary for efficient placentation and successful pregnancy. A 10-year prospective population study revealed that there is an increased rate of fetal loss and reduced overall fertility among couples with matching HLA haplotypes or loci (27). Furthermore, in pregnant women it has been shown that the more HLA-A, -B, -C, -DRB1 and -DQB1 alleles are shared with the fetus, the higher the risk of preeclampsia (28). Nonetheless, the risk declines among women with normal to high exposure to paternal seminal fluid, suggesting that frequent intercourse before conceiving favors successful pregnancy (28-30).
All in all, although we think we select our partner based on an attractive face, charisma or wittiness, without us knowing, it also comes down to the fact that we are instructed by our instincts and our subconscious needs for healthy procreation. Especially women are led by finding an HLA dissimilar partner, most likely because women feel subconsciously a bigger need to invest in healthy offspring. Unfortunately, nowadays these instinctive drives can be disrupted by external factors, since for example use of the contraceptive pill can alter partner choice (31). So before going on a date, instead of only checking his or hers Facebook profile, check the HLA profile as well, it might prevent future heartbreak.
Reference list
- Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. 2014;15(6):379-93.
- Langefors A, Lohm J, Grahn M, Andersen O, von Schantz T. Association between major histocompatibility complex class IIB alleles and resistance to Aeromonas salmonicida in Atlantic salmon. Proc Biol Sci. 2001;268(1466):479-85.
- Hedrick PW, Parker KM. MHC VARIATION IN THE ENDANGERED GILA TOPMINNOW. Evolution. 1998;52(1):194-9.
- Paterson S, Wilson K, Pemberton JM. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population. Proc Natl Acad Sci U S A. 1998;95(7):3714-9.
- Ma Y, Yuan B, Yi J, Zhuang R, Wang J, Zhang Y, et al. The genetic polymorphisms of HLA are strongly correlated with the disease severity after Hantaan virus infection in the Chinese Han population. Clin Dev Immunol. 2012;2012:308237.
- Nguyen TP, Kikuchi M, Vu TQ, Do QH, Tran TT, Vo DT, et al. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Negl Trop Dis. 2008;2(10):e304.
- Prugnolle F, Manica A, Charpentier M, Guégan JF, Guernier V, Balloux F. Pathogen-driven selection and worldwide HLA class I diversity. Curr Biol. 2005;15(11):1022-7.
- Thomsen D, Erikstrup C, Jespersen S, Medina C, Té DDS, Correira FG, et al. The influence of human leukocyte antigen-types on disease progression among HIV-2 infected patients in Guinea-Bissau. AIDS. 2018;32(6):721-8.
- Doherty PC, Zinkernagel RM. A biological role for the major histocompatibility antigens. Lancet. 1975;1(7922):1406-9.
- Doherty PC, Zinkernagel RM. Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature. 1975;256(5512):50-2.
- Kekäläinen J, Vallunen JA, Primmer CR, Rättyä J, Taskinen J. Signals of major histocompatibility complex overdominance in a wild salmonid population. Proc Biol Sci. 2009;276(1670):3133-40.
- McClelland EE, Penn DJ, Potts WK. Major histocompatibility complex heterozygote superiority during coinfection. Infect Immun. 2003;71(4):2079-86.
- Penn DJ, Damjanovich K, Potts WK. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc Natl Acad Sci U S A. 2002;99(17):11260-4.
- Hraber P, Kuiken C, Yusim K. Evidence for human leukocyte antigen heterozygote advantage against hepatitis C virus infection. Hepatology. 2007;46(6):1713-21.
- Yamazaki K, Beauchamp GK, Curran M, Bard J, Boyse EA. Parent-progeny recognition as a function of MHC odortype identity. Proc Natl Acad Sci U S A. 2000;97(19):10500-2.
- Milinski M, Croy I, Hummel T, Boehm T. Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment. Proc Biol Sci. 2013;280(1755):20122889.
- Penn DJ, Potts WK. The Evolution of Mating Preferences and Major Histocompatibility Complex Genes. Am Nat. 1999;153(2):145-64.
- Freeman-Gallant CR, Meguerdichian M, Wheelwright NT, Sollecito SV. Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Mol Ecol. 2003;12(11):3077-83.
- Kromer J, Hummel T, Pietrowski D, Giani AS, Sauter J, Ehninger G, et al. Influence of HLA on human partnership and sexual satisfaction. Sci Rep. 2016;6:32550.
- Hämmerli A, Schweisgut C, Kaegi M. Population genetic segmentation of MHC-correlated perfume preferences. Int J Cosmet Sci. 2012;34(2):161-8.
- Milinski M, Wedekind C. Evidence for MHC-correlated perfume preferences in humans.: Behavioral Ecology; 2001. p. 140-9.
- Thornhill R, Gangestad S, Miller R, Scheyd G, McCollough J, Franklin M. Major histocompatibility complex genes, symmetry, and body scent attractiveness in men and women. Behav Ecol. 2003;14(5):668-78.
- Fromhage L, Kokko H, Reid JM. Evolution of mate choice for genome-wide heterozygosity. Evolution. 2009;63(3):684-94.
- Winternitz J, Abbate JL, Huchard E, Havlíček J, Garamszegi LZ. Patterns of MHC-dependent mate selection in humans and nonhuman primates: a meta-analysis. Mol Ecol. 2017;26(2):668-88.
- Garver-Apgar CE, Gangestad SW, Thornhill R, Miller RD, Olp JJ. Major histocompatibility complex alleles, sexual responsivity, and unfaithfulness in romantic couples. Psychol Sci. 2006;17(10):830-5.
- Wedekind C, Seebeck T, Bettens F, Paepke A. MHC-Dependent Mate Preferences in Humans. Proc R Sac Land. 1995;260(1359):245-9.
- Ober C, Hyslop T, Elias S, Weitkamp LR, Hauck WW. Human leukocyte antigen matching and fetal loss: results of a 10 year prospective study. Hum Reprod. 1998;13(1):33-8.
- Triche EW, Harland KK, Field EH, Rubenstein LM, Saftlas AF. Maternal-fetal HLA sharing and preeclampsia: variation in effects by seminal fluid exposure in a case-control study of nulliparous women in Iowa. J Reprod Immunol. 2014;101-102:111-9.
- Koelman CA, Coumans AB, Nijman HW, Doxiadis II, Dekker GA, Claas FH. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. 2000;46(2):155-66.
- Nederlof I, Meuleman T, van der Hoorn MLP, Claas FHJ, Eikmans M. The seed to success: The role of seminal plasma in pregnancy. J Reprod Immunol. 2017;123:24-8.
- Alvergne A, Lummaa V. Does the contraceptive pill alter mate choice in humans? Trends Ecol Evol. 2010;25(3):171-9.






